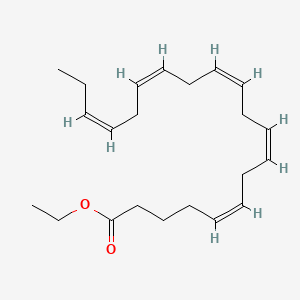
Cardiovascular risk reduction
Adult: As an adjunct to statin therapy in patients with triglyceride levels ≥150 mg/dL, established CV disease, or type 2 diabetes mellitus, and at least 1 CV risk factor: 4 g daily taken as 2 g bid. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Severe hypertriglyceridaemia
Adult: As an adjunct to diet in patients with triglyceride levels ≥500 mg/dL: 2 g bid. Treatment guidelines may vary among countries or individual products. Refer to specific product guidelines.




 Sign Out
Sign Out